Explore our network of country and industry based websites to access localized information, product offerings, and business services across our group.
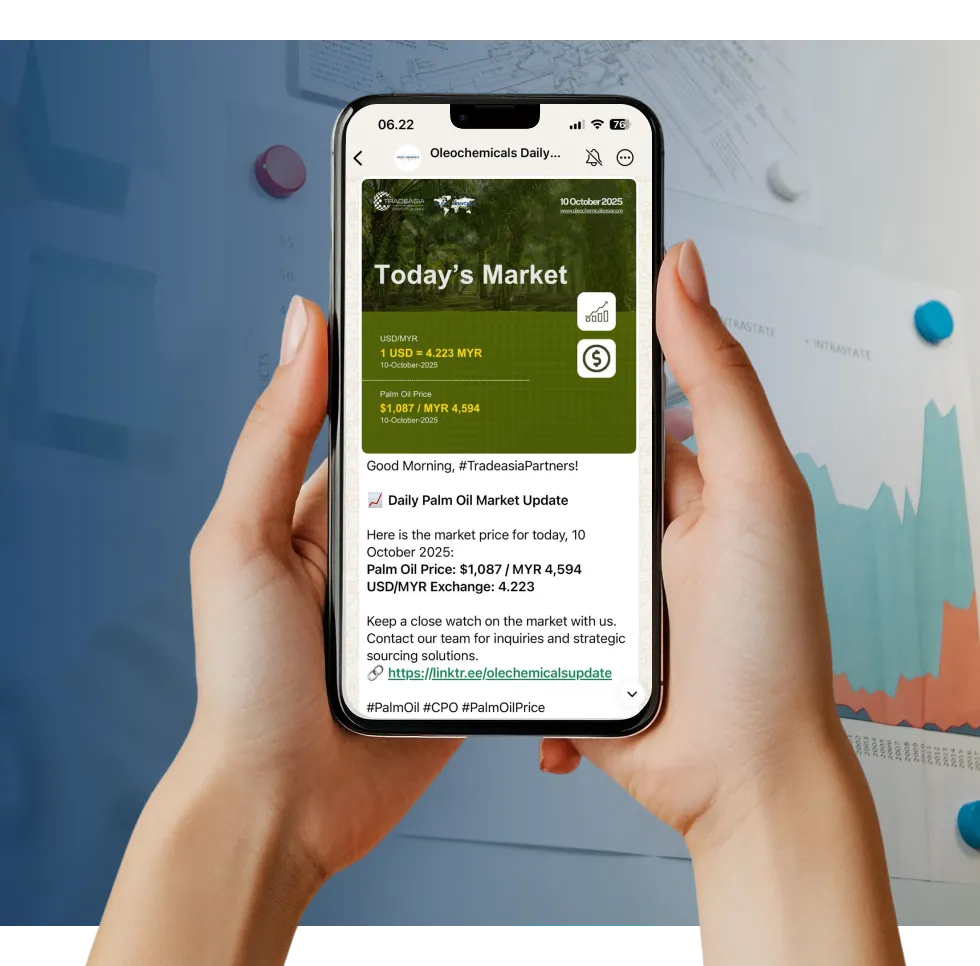
Access reliable chemical market information through our update channels.
Real-time Updates
Daily Updates
Log in to start sending quotation requests for any product.
Don't have an account? Sign Up Here
Log in now to access technical product documents from our product range.
Don't have an account? Sign Up Here
Home All Products Tartaric Acid
|
IUPAC Name |
: 2,3-Dihydroxybutanedioic Acid |
|
Cas Number |
: 87-69-4 |
|
HS Code |
: 2918.12.00 |
|
Formula |
: HOOC(CHOH)2COOH |
|
Appearance Name |
: White Crystalline Powder |
|
Common Names |
: Tartaric Acid |
|
Packaging |
: 25 kg HDPE bag, 25 kg paper bag |
For more detailed information including pricing, customization, and shipping:
Tartaric acid is a white crystalline diprotic aldaric acid. It is predominantly found naturally in several plants, particularly bananas, grapes, and tamarinds. It is a useful raw material in organic chemistry for synthesizing other chiral molecules.
Tartaric acid is obtained from a naturally-occurring component of lees, a solid by-product of fermentation. The former by-products mostly consist of potassium bitartrate (KHC4H4O6). This potassium salt is converted to calcium tartrate (CaC4H4O6) upon treatment with milk of lime (Ca(OH)2):
KO2CCH(OH)CH(OH)CO2H + Ca(OH)2 → Ca(O2CCH(OH)CH(OH)CO2) + KOH + H2O
Higher yields of calcium tartrate are obtained with the addition of calcium chloride. Calcium tartrate is then converted to tartaric acid by treating the salt with aqueous sulfuric acid.
This process yields tartaric acid:
Ca(O2CCH(OH)CH(OH)CO2) + H2SO4 → HO2CCH(OH)CH(OH)CO2H + CaSO4
Tartaric acid is widely used as an acidulant in beverages and other food products, such as soft drinks, wine, candy, bread, and some colloidal sweetmeats. Tartaric acid is also commonly combined with baking soda to function as a leavening agent in recipes and is one of the main acids found in wine. It is added to give a sour taste and is used as an antioxidant under E number E 334.
Tartaric acid is used with citric acid in effervescent salt production, improving oral medications' taste. The potassium antimonyl derivative of the acid known as tartar emetic is included, in small doses, in cough syrup as an expectorant.
Tartaric acid has several applications for agricultural use, such as a chelating agent. Its chelating properties on metal ions drive its popularity in the farming industry for purposes such as complexing micronutrients in soil fertilizer.
Tartaric acid is used in the metal industries as a chelating agent for cleaning metal surfaces consisting of aluminum, copper, iron, and alloy of these metals. Its reduction properties can be used chemically as a reductive agent in manufacturing mirrors or as an imaging agent in photography. Its acidity can also be used as a catalyst in the resin finishing of polyester fabric or pH value regulator in oryzanol production.

